
Appropriate controls are crucial for the evaluation of staining specificity. Negative controls are essential to identify false positive staining.
SYSY recommends performing the following staining controls to support the specificity of your stainings.
When using chromogenic detection in IHC, endogenous peroxidases or alkaline phosphatases can produce false positive staining when not blocked appropriately. Omitting the primary antibody and the secondary system in the staining protocol will reveal endogenous enzyme activity. Use 3% H2O2 to block endogenous peroxidases. Endogenous alkaline phosphatases can be blocked with 1 mM Levamisole.
Other false positive signals inheritent to the tissue itself that can be easily confounded with the DAB signal due to yellow-brown color are: hemosiderine (e.g. liver, spleen, and bone marrow), bilirubin (liver) lipofuscin (e.g. nerve cells, heart, and liver), and melanin in corneal endothelial cells (brown eyes) and human skin cells.
In fluorescent IHC-P, tissue autofluorescence may be confused with specific signals or unspecific background.
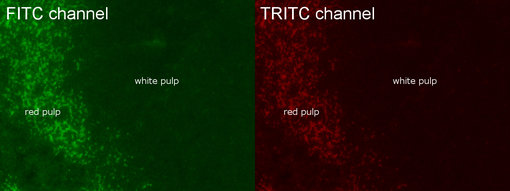
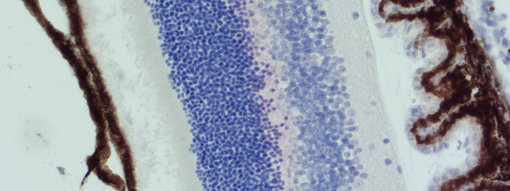
Figure 1: Autofluorescence in FFPE mouse spleen in FITC and TRIC channel. Autofluorescence is mainly seen in red pulp.
Figure 2: Melanin (brown) in mouse retina stained with AP-RED.
Negative controls omitting the primary antibody are necessary to identify false positive staining from the secondary system.
False positive staining can occur from non-specific binding of the secondary antibody. Use a secondary antibody that has been pre-adsorbed against the same species your tissue sample originated from (e.g. use a mouse-adsorbed anti-rat secondary antibody when detecting a rat primary antibody in mouse tissue).
When using mouse primary antibodies on mouse tissues, endogenous mouse IgG staining can be reduced using a M.O.M. kit.
False positive staining can occur from endogenous biotin when using the ABC method. Block endogenous biotin with an excess of unlabeld (Strept-)Avidin followed by blocking remaining (Strept-)Avidin sites with free biotin (e.g. Avidin-Biotin Kit).
Use tissue types that are known to express high levels of the protein of interest. This control will verify that your staining protocol is working.
Use tissue types that are known to NOT express the protein of interest. This may be tissue samples from other species when species specific antibodies are used. Tissue from knock-out or knock-down models are particularly useful.