
Multiplex IHC enables the detection of two or more targets on one slide and is particulaly valuable when single IHC-P is not sufficient to accurately show the anticipitated result: e.g. immunophenotyping of cells using two or more markers. Multiplex IHC-P can be done using either fluorophores or chromogenic substrates. However, Multiplexing has many technical challenges, so several important issues should be considered:
When using indirect IHC methods for antibody-antigen detection (see: Secondary-systems), the primary antibodies should ideally originate from different host species to avoid cross-reaction of the secondary systems. Monoclonal antibodies from the same host species but with different isotypes can be detected using isotype-specific secondary antibodies. In chromogenic IHC-P, heat or low pH-buffers may be used to strip antibodies from the first IHC-P round when only primary antibodies from the same species and isotype are available. When including a heat denaturation step in your protocol, consider taking a heat-resistant substrate, e.g. DAB.
In Immunofluorescence, use fluorophores with narrow emission spectra, narrow bandpass filters in your microscope to avoid bleed-through, and target the less abundant protein with the brighter fluorophore. Autofluorescence of the tissue may hamper interpretation of results especially in green- and red-channel fluorophores. Include a control slide without antibody and fluorophore to check for autofluorescence in your tissue (see: IHC-Controls).
Spectral differentiation of chromogenic substrates may be difficult, especially for co-localized targets. The mixed color should be well contrasted from single stains. Pay attention to mounting requirements of chromogens and use substrates with higher sensitivity for less abundant target proteins.
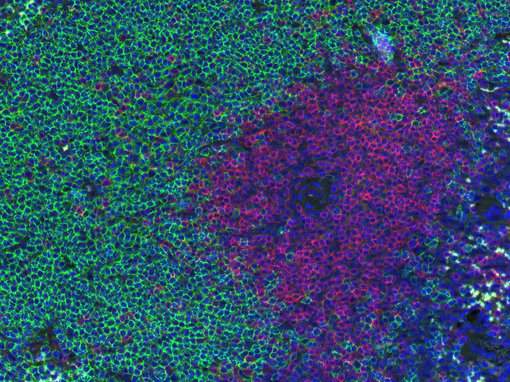
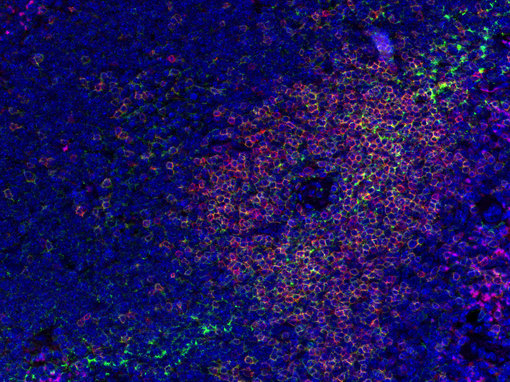
Figure 1: Doublestaining of B-cells (CD19, green) and T-cells (CD3e, red) in mouse spleen.
Figure 2: Doublestaining of a general T-cell marker (CD3e, red) with anti-CD4 (green) in mouse spleen.
Secondary antibodies may cross react with endogenous immunoglobulins in the tissue or produce false-positive staining through non-specific binding of the secondary antibody. In Multiplex IHC-P, secondary antibodies can furthermore cross-react with each other. Use secondary antibodies that have not only been pre-adsorbed against Igs of the species to be stained, but also IgGs of the host species of the other secondary antibodies used in the Multiplex protocol. In addition to the controls depicted in IHC-Controls, Multiplex experiments should include control slides where only one primary antibody is omitted in the staining protocol (see Figure 1).
Figure 3 shows the establishment of a Multiplex protocol for anti-mouse and anti-human Ki67 in mouse spleen engraftet with human CD34+ cells (A1). The specificity of the primary antibodies are verified using positive and negative controls (wild-type mouse spleen, human tonsil). Putative cross-reactivity of secondary systems is verified by omitting either anti-human (column 2) or anti-mouse Ki67 (column 3) in the staining protocol.
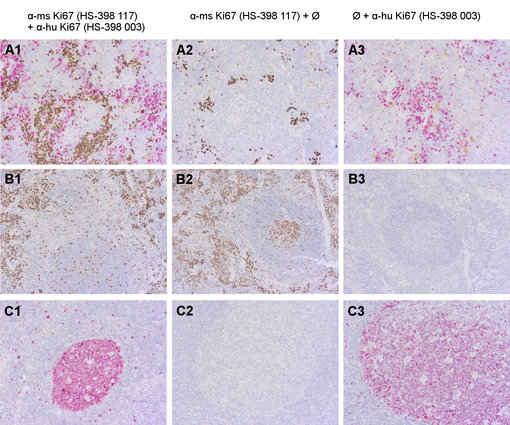
Figure 3: Doublestaining of formalin-fixed paraffin embedded murine (A1-3: mouse spleen engrafted with human CD34+-cells; B1-3: wildtype mouse spleen) and human (C1-3: human tonsil) tissues using rabbit anti-human Ki67 (cat. no. HS-398 003; AP-RED, red color) and rat anti-mouse Ki67 (cat. no. HS-398 117; DAB, brown color).
Figure 3: Doublestaining of formalin-fixed paraffin embedded murine (A1-3: mouse spleen engrafted with human CD34+-cells; B1-3: wildtype mouse spleen) and human (C1-3: human tonsil) tissues using rabbit anti-human Ki67 (cat. no. HS-398 003; AP-RED, red color) and rat anti-mouse Ki67 (cat. no. HS-398 117; DAB, brown color).
When primary antibodies require different antigen retrieval conditions to produce optimal staining results, small-scale single stain IHC-P test runs comparing different protocols need to be done in advance to find the most compatible antigen retrieval condition for all primary antibodies.
Multiplex IHC-P protocols can be designed as sequential staining or as simultaneous staining. In sequential staining protocols, one staining procedure succeeds another. When performing the first staining using DAB as the chromogen, pay attention to the DAB 'shielding' or 'sheltering' effect - the brown DAB precipitate may shield other targets needed for the second IHC-P staining. The advantage of this protocol is that stripping steps can be included to avoid cross-reactivity of secondary antibodies. In simultaneous staining protocols, which are mainly used in immunofluorescence, the primary antibodies are applied simultaneously. This protocol is less time-consuming, but can negatively influence antibody performance in chromogenic protocols due to increased washing steps. When establishing Multiplex IHC-P protocols, always compare the outcome of the individual antibodies to their performance in single stains.