
Immunohistochemistry (IHC) has become an essential tool for clinical and translational research and diagnostic pathology. Intensity and tissue distribution of immunohistochemical staining have major impact on diagnosis and therapeutic opportunities in clinical pathology. Prognostic biomarkers, e.g. Ki67 expression in breast cancer (Yamamoto et al., 2013), provide information on the likely patient health outcome. Predictive biomarkers indicate the likely benefit of the patient from a specific treatment, e.g. Her2 overexpression and amplification are predictive of response to Trastuzumab (Hofmann et al., 2008). But also research projects are dependent on reproducible results.
Many tissue processing parameters have been identified to affect IHC-P staining results:
SYSYs standard tissue preparation protocol comprises a 24 hrs fixation step in neutral buffered formalin. However, customers may use other fixation protocols. Therefore, we evaluated the influence of formalin fixation duration on immunohistochemical antigen detection in mouse spleen using three different time points: 13 hrs (presumed under-fixation), 24 hrs (standard fixation) and 48 hrs (presumed over-fixation). Eight HistoSure antibodies directed against common murine immune cell subtypes were tested, respectively.
Staining was performed according to the affiliated HistoSure IHC-P reference protocols. Staining intensity of 3-4 independent experiments was evaluated using a 4-point scoring system (Staining Intensity Score, SIS): 0 (no staining), 1 (light staining), 2 (medium staining) or 3 (dark staining).
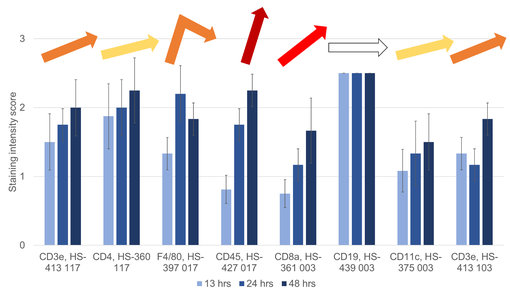
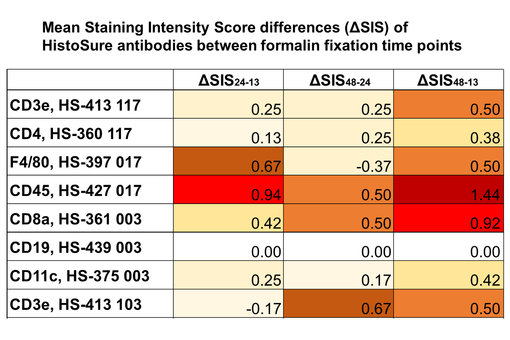
Figure 1: Staining Intensity Scores (SISs) of HistoSure Antibodies in sections of mouse spleen fixed for either 13 hrs (light blue), 24 hrs (blue) or 48 hrs (dark blue) in neutral buffered formalin. Steepness and color of arrows reflect the difference in Staining Intensity Scores between formalin fixation timepoints 13 hrs and 48 hrs. white = no difference; yellow = minor difference; red = major difference.
Figure 2: Comparision of mean Staining Intensity Score (SIS) differences between formalin fixation timepoints (ΔSIS24-13: difference of mean SIS at 24 hrs and mean SIS at 13 hrs; ΔSIS48-24: difference of mean SIS at 48 hrs and mean SIS at 24 hrs; ΔSIS48-13: difference of mean SIS at 48 hrs and mean SIS at 13 hrs) using a heat map. white = no difference; yellow = minor difference; red = major difference.
In general, IHC-P staining was more sensitive to presumed under-fixation at 13 hrs timepoint (figure 1 and 2). The average Staining Intensity Score differed by 0.33 between the 13 hrs and the 24 hrs fixation time point and by 0.25 between the 24 hrs and the 48 hrs fixation time point. Anti-CD45 staining (figure 1 and 3) and anti-CD8a staining (figure 1) were strongly affected from variations in formalin fixation duration in our study, indicated by red or dark red color in the heat map at SIS48-13 (figure 3; last column). Only anti-CD19 staining was completely unaffected from variations in formalin fixation duration (figure 4).
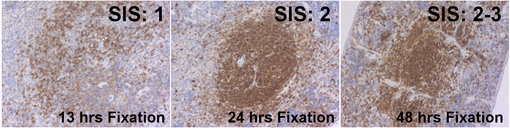
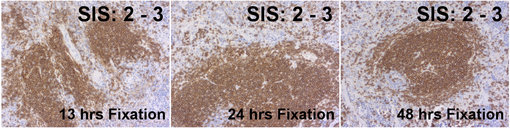
Figure 3: Immunohistochemical staining of murine spleens fixed for 13 hrs, 24 hrs or 48 hrs in neutral buffered formalin with rat anti-CD45 HS-427 017. The Staining Intensity Score increases from 1 to 2.5 with prolongued duration of formalin fixation.
Figure 4: Immunohistochemical staining of murine spleens fixed for 13 hrs, 24 hrs or 48 hrs in neutral buffered formalin with rabbit anti-CD19 HS-439 003. The Staining Intensity Score is equal at all formalin fixation time points.
Although most HistoSure antibodies showed superior results in the mouse spleen fixed for 48 hrs, anti-F4/80 showed optimal staining results in the mouse spleen fixed for 24 hrs using SYSYs recommended staining protocol (figure 1 and 2).
Thus, adequate formalin fixation is essential for the staining outcome. Insufficient fixation will not only cause loss of immunohistochemical antigenicity, but can also lead to inferior tissue morphology. Also prolongued fixation may negatively affect Staining Intensity Scores. Therefore, standardization of formalin fixation duration is essential for reproducibility of IHC-P experiments. SYSY recommends tissue fixation in neutral buffered formalin for 24 hrs.